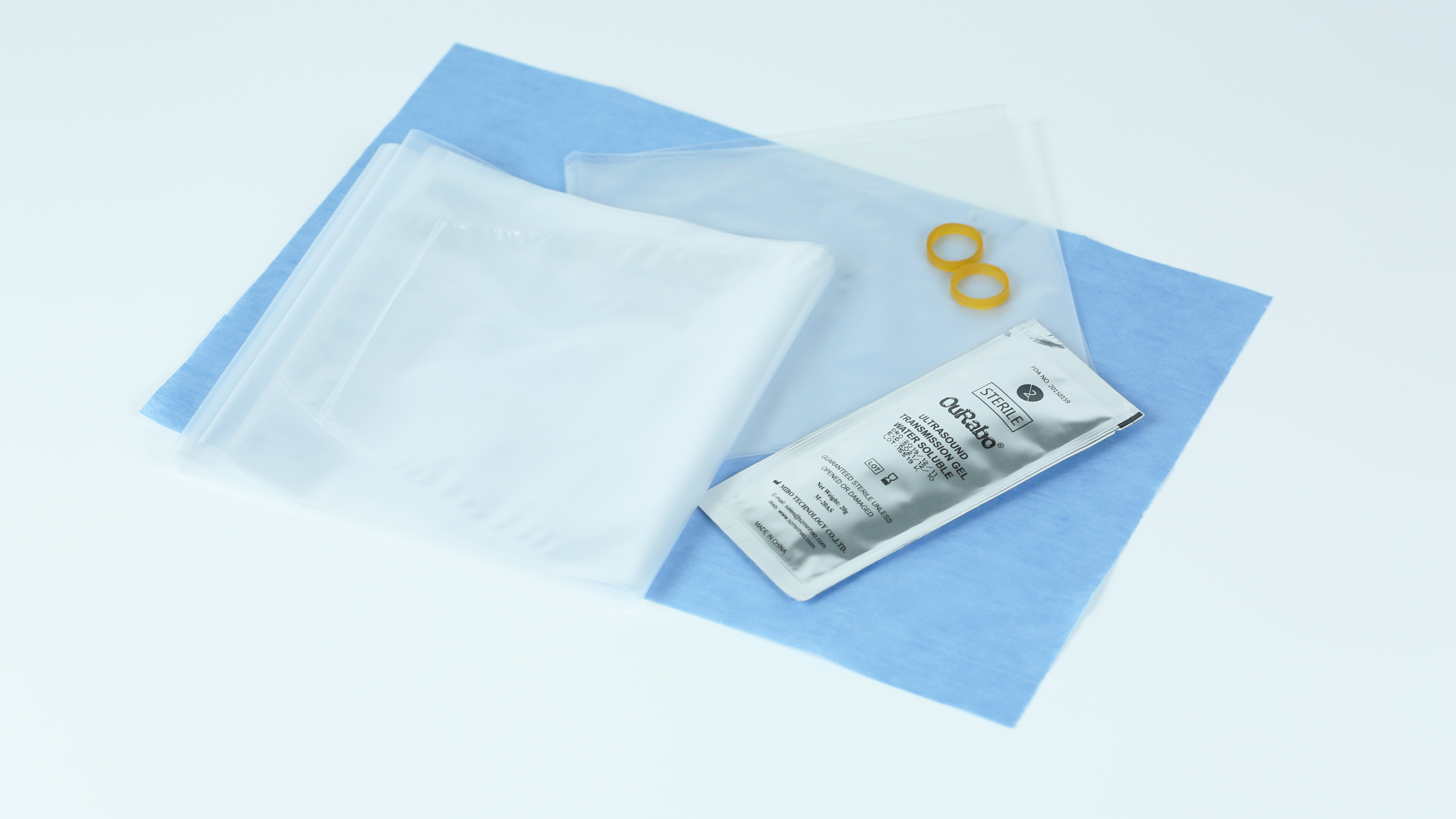
| Product | Description |
|---|---|

SG2003 |
Sterile PE film 15.2cm tapered to 7.6244cm, TPU film 1430cm, Flat Folding, w/20g gel, Single Use |

SG2002 |
Sterile PE film 15.2cm tapered to 7.6244cm, TPU film 1430cm, Accordion. Folding, w/o gel, Single Use |

SG2004 |
Sterile TPU film 10*15cm, Flat Folding, w/20g gel, Single Use |

SG2005 |
Sterile TPU film 8*12cm, Flat Folding, w/20g gel, Single Use |

SG2006 |
Sterile TPU film 10*25cm, Flat Folding, w/20g gel, Single Use |

SG2001 |
Sterile PE film 15.2cm tapered to 7.6244cm, TPU film 1430cm, Accordion. Folding, w/20g gel, Single Use |
Certification
ISO 13485:2016
Medical devices — Quality management systems — Requirements for regulatory purposes
International Organization for Standardization (ISO)
Certification
EN ISO 13485:2016
Medical devices — Quality management systems — Requirements for regulatory purposes
International Organization for Standardization (ISO)
Certification
93/42/EEC Annex II
COUNCIL DIRECTIVE 93/42/EEC of 14 June 1993 concerning medical devices
Emergo: Global Medical Device Consulting
Connect with Disposable Ultrasound Probe Cover through Junala
Junala is a global platform connecting medical device manufacturers with distributors with over XX years of experience.
Over 215+ Years of Healthcare Experience
The team at Junala can bring the right expertise to help you select the right products.
Contact Us
To give us a better understanding of your needs, we would like to know more about your business needs and what you are looking for from a strategic partner moving forward.